Impact of changing groundwater level on nutrient mobility
Abstract¶
One of the major concerns in rewetted wetlands is the increase of nutrient concentration in groundwater and the possible contamination of adjacent aquatic ecosystems (i.e. eutrophication). The increase of phosphorus solubility (mobilization) and diffusion are indeed the most important changes of rising groundwater levels. But also, the increase of polluting gases like methane, which may hamper climate change mitigation purposes.
With an increase in groundwater levels, and hence soil moisture, soils shift to anaerobic conditions because of the lack of oxygen. In these conditions, specialized soil bacteria dominate and break down the organic matter at a slow rate. Decomposition of the organic matter (mineralization) into mainly methane and carbon dioxide, and nutrient intake (assimilation), are therefore very slow processes, which cause plant residue accumulation. At the same time, changes in pH alter the amount of soluble nutrients and chemicals in the soil. Inorganic phosphorous is usually bonded to clay particles, iron, aluminum and calcium. The altered pH in anaerobic conditions increases the solubility of these elements, and the adsorbed phosphorous and organic carbon substances are released to the soil solution. Nitrogen on the other hand is mostly lost from the soil as nitrogen gas. These nitrogen losses cause less soluble nitrogen to be available for plants.
Introduction¶
Rising groundwater levels causes drastic changes in the ecosystem, and to the physical and electrochemical characteristics of the soil Harpenslager et al., 2015. Depending on the intensity of rewetting, conventional agriculture may no longer be possible. The increase in nutrient mobilization in soils previously under agricultural use can cause contamination of the surface water, with undesirable effects in aquatic environments (i.e. eutrophication) Zak & Gelbrecht, 2007Johnston et al., 2005. Rewetting of drained agricultural peatlands is especially critical due to the high amount of nutrients and organic matter content Zak & Gelbrecht, 2007. Numerous studies highlight the capacity of wetland vegetation like cattail (Thypa sp.) to remove excess nitrogen and phosphorous from surface and pore water. As such, they prevent nutrient accumulation and transport, and mitigate methane (CH4) emissions, which are known to increase in flooded conditions Vroom et al., 2018Belle, 2021Geurts et al., 2020.
The extent and duration of soil under saturated conditions, and the presence of microbiological activity, define whether waterlogged conditions (anaerobic conditions) may occur Moore et al., 1998. These conditions are present in rewetted wetlands, where the water level is kept above or slightly under ground surface. The oxygen from the underlying water column can reach a small portion of soil surface before it gets depleted, creating a thin aerobic layer on top of a thicker anaerobic layer. The depth of this aerobic layer depends on the oxygen supply and the consumption rate in the soil, being even inexistent in soils with high decomposable organic matter at the soil surface Buresh et al., 2008. Therefore, the chemical processes in the anaerobic layer are the main interest in this chapter.
In Flanders, both soil phosphorus (P) and nitrogen availability are high due to intensive livestock farming and agriculture. Substantial P-reserves have been developed over the years in the Flemish farmland, and nitrogen emissions (mainly ammonia) from agricultural activities have remained alarming, being the focal point of manure and fertilizers legislations Bomans et al., 2005Departement Omgeving, 2022. Phosphorous is normally very strongly bound to soil particles, erosion and surface runoff are the main transport mechanisms of particulate P to surface waters Bomans et al., 2005. Under anaerobic conditions, part of the bounded phosphorus enters the soil solution and can be transported through surface runoff and also as soluble or dissolved P Ponnamperuma, 1972, with a serious risk of eutrophication in nearby water bodies. Similarly, excessive nitrogen emissions and later deposition in the soil cause eutrophication and soil acidification Departement Omgeving, 2022. It is therefore important to investigate the possible impacts of rising groundwater levels on nutrient mobilization, gas emissions and availability.
This chapter will describe the main chemical and physical transformations of nutrients and other linked elements in waterlogged soils (anaerobic conditions) with a main focus on phosphorus, and their possible impacts on the ecosystem.
Chemistry of submerged soils under anaerobic conditions¶
A rising water table causes a fast shift from aerobic to anaerobic soil processes Harpenslager et al., 2015. Under anaerobic conditions, soils are in a reduced state instead of an oxidized state, which cause several electrochemical changes such as decrease in redox potential, changes in pH, drastic shifts in mineral equilibria, and sorption and desorption of ions (Figure 1) Ponnamperuma, 1972. Reduction is the gain of electrons by an oxidizing agent or acceptor while oxidation is the loss of electrons by a reducing agent or donor. Redox potential represents how easily electrons are transferred to or from chemical components in a solution. Under anaerobic conditions, facultative and obligate anaerobic microorganisms use the organic matter as substrate or donor, and oxidized soil components like nitrate (NO3-) or manganese dioxide (MnO2) as electron acceptors in their respiration. The reduced components during anaerobic respiration are normally carbon dioxide (CO2), methane (CH4), ammonium (NH4+), ammonia (NH3), hydrogen sulfide (H2S), ethylene (C2H4) and other residues Ponnamperuma, 1972. Under anaerobic conditions, soil has a low oxidation-reduction potential because anaerobic bacteria work at low energy levels due to incomplete decomposition of carbohydrates Zak & Gelbrecht, 2007Ponnamperuma, 1972Buresh et al., 2008. Therefore, decomposition and assimilation are very slow processes and plant residues accumulate in waterlogged or poorly drained soils (e.g. peatlands).
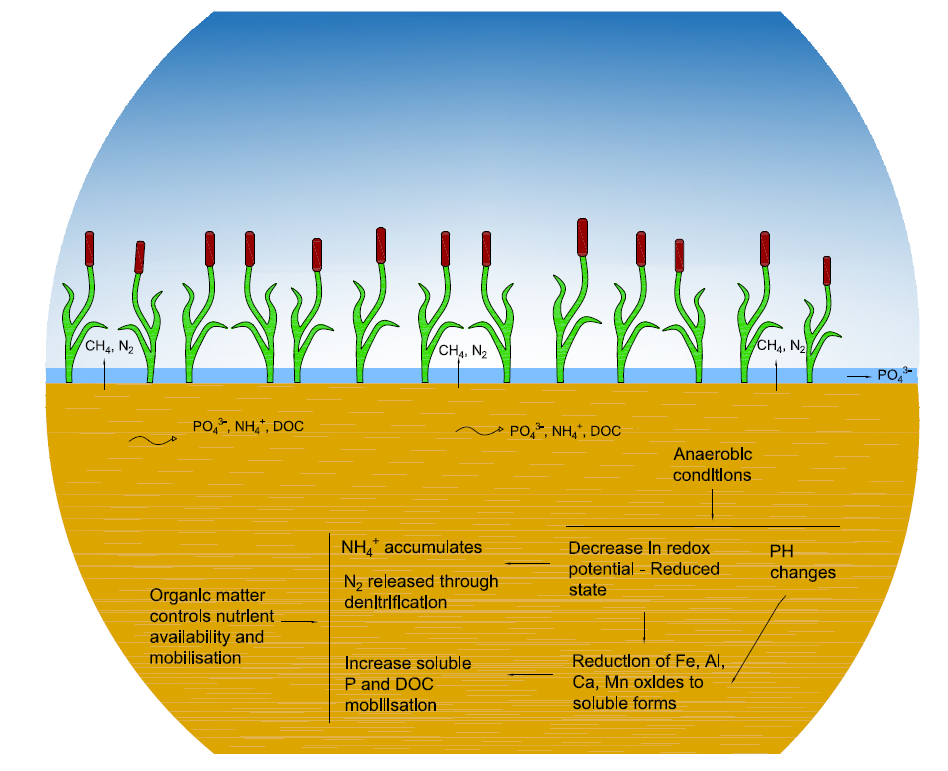
Figure 1:Main electrochemical transformations in soil under submerged conditions.
Sequential reduction¶
During the shift from aerobic to anaerobic conditions, oxygen is quickly depleted and other soil compounds are used as electron acceptors for anaerobic bacteria. The choice of these electron acceptors occurs roughly in the sequence shown in Table %s, according to the redox potential or electron activity (pe) Ponnamperuma, 1972Amery, 2012. The more negative pe is, the stronger the reduction potential is.
After oxygen depletion, MnO2 (Mn4+) or NO3- are first reduced to Mn2+ and N2 respectively.
NO3- reduction begins only after the oxygen concentration has dropped to a very low value, and their presence slow down the reduction of the next oxides in the sequence. MnO2 has less influence than NO3- because its insolubility in water and it is used as electron acceptor by only a few distinct bacteria. Next in the sequence is ferric iron Fe(OH)3 (Fe3+), which is reduced to ferrous iron (Fe2+). Under very reduced conditions, sulphate (SO42-), CO2 and H+ are used as electron acceptors for bacterial respiration Ponnamperuma, 1972Boyd, 1995.Table 1:Sequence of half reduction reactions in submerged soils with pe values predicted for equal concentrations of the reduced and oxidized species in solution Amery, 2012
pe* | ||
pH 5 | pH 7 | |
1) 1/4O2+ e-+H+=1/2H2O | 15.6 | 13.6 |
2) 1/2MnO2+ e-+2 H+=1/2Mn2++H2O | 12.8 | 8.8 |
3) 1/2NO3-+ e-+ H+=1/2 NO2-+1/2 H2O | 9.3 | 7.3 |
4) Fe(OH)3+ e-+3 H+=Fe2++3H2O | 4.8 | -1.2 |
5) 1/8SO4 2-+ e-+5/4 H+=1/8H2S+1/2H2O | -1.0 | -3.5 |
6) 1/8CO2 + e -+ H+=1/8CH4+1/4H2O | -2.1 | -4.1 |
7) H++ e-=1/2H2 | -5.0 | -7.0 |
8) 1/4CO2+ e-+H+=1/4CH2O+1/4H2O | -6.1 | -8.1 |
*for solid-phase/solution equilibria: concentrations of dissolved species of 10-4 M. Atmospheric gas composition assumed: partial pressure of O2 of 0.21 bar, N2 of 0.778 bar, and CO2 of 3.2 10-4 bar. | ||
Oxygen and other gases¶
In saturated conditions, oxygen and other gases can transport through the soil only by molecular diffusion in the interstitial water. This process is much slower than diffusion through gas-filled pores (about 10000 times slower), and therefore the oxygen diffusion rate falls sharply when the soil reaches saturation. In just few hours after saturation, all the available molecular oxygen present in the water or trapped in the soil is depleted by microorganisms. In these anaerobic conditions, apart from carbon dioxide (CO2), other intermediate gases like methane (CH4) and ethylene (C2H4) are also produced during respiration and alcoholic fermentation, and accumulate in the soil Moore et al., 1998.
pH¶
Soil pH is a measure of the concentration of hydrogen ions in the soil solution. Its value ranges from 0 to 14, being 5.5 to 8.0 considered ideal for plant growth. Soil with low pH values (<5.5) are considered acidic, while soils with high pH values (>8.0) are classified as alkaline Rengasamy, 2022. pH increases in saturated acidic soils while it decreases in saturated alkaline soils, until both reach a relatively stable value of about 7 Ponnamperuma, 1972. This can be seen in several experiments done in rewetting projects Zak & Gelbrecht, 2007Riet et al., 2013. The increase in pH in acidic soils has to do with soil reduction processes while its decrease in alkaline soils is because CO2 accumulation. However, this phenomenon can change depending on the organic matter and iron contents. Organic matter and iron enhance the pH decrease in basic or alkaline soils while restricting the increase of pH in acidic soils Ponnamperuma, 1972.
pH highly influences the hydroxide, carbonate, sulfide, phosphate, and silicate equilibria in submerged soils. This equilibria regulates their chemical and physical transformations, including precipitation and dissolution of solids, sorption and desorption of ions Ponnamperuma, 1972; and ultimately, their availability in the soil solution, and for the plants.
Temperature¶
Soil temperature mainly determines the oxygen depletion rates in submerged soils. At low temperature, the biological activity of plants and soil microorganisms is low and therefore the oxygen demand is also low. The oxygen demand however increases exponentially with temperature. At higher temperature, plants grow faster and microbiological activity increases, causing a fast oxygen depletion in warm conditions Moore et al., 1998. Temperature has therefore a strong effect on soil reduction processes in flooded soils Ponnamperuma, 1972.
Phosphorous transformation¶
In the soil, phosphorus (P) is present attached to soil particles, as minerals like Fe-Al oxides and Ca-carbonates, as part of organic matter and a very small percentage dissolved in the soil solution Bomans et al., 2005. Phosphorus (P) is a limiting nutrient for crop growth, especially during the vegetative stage. In Flanders, critical P values range from 59 mg P/kg dry soil in winter wheat up to 164 mg P/kg dry soil in maize Stijn Martens et al., 2020. Soluble forms of P like orthophosphates ions ( H2PO4- and HPO42-)
are absorbed by the plants and allocated to the fruits and seeds during reproductive stages Bomans et al., 2005. Excess soluble P is lost by surface runoff and leaching. Figure 2 explains the main transformations of phosphorous in the soil.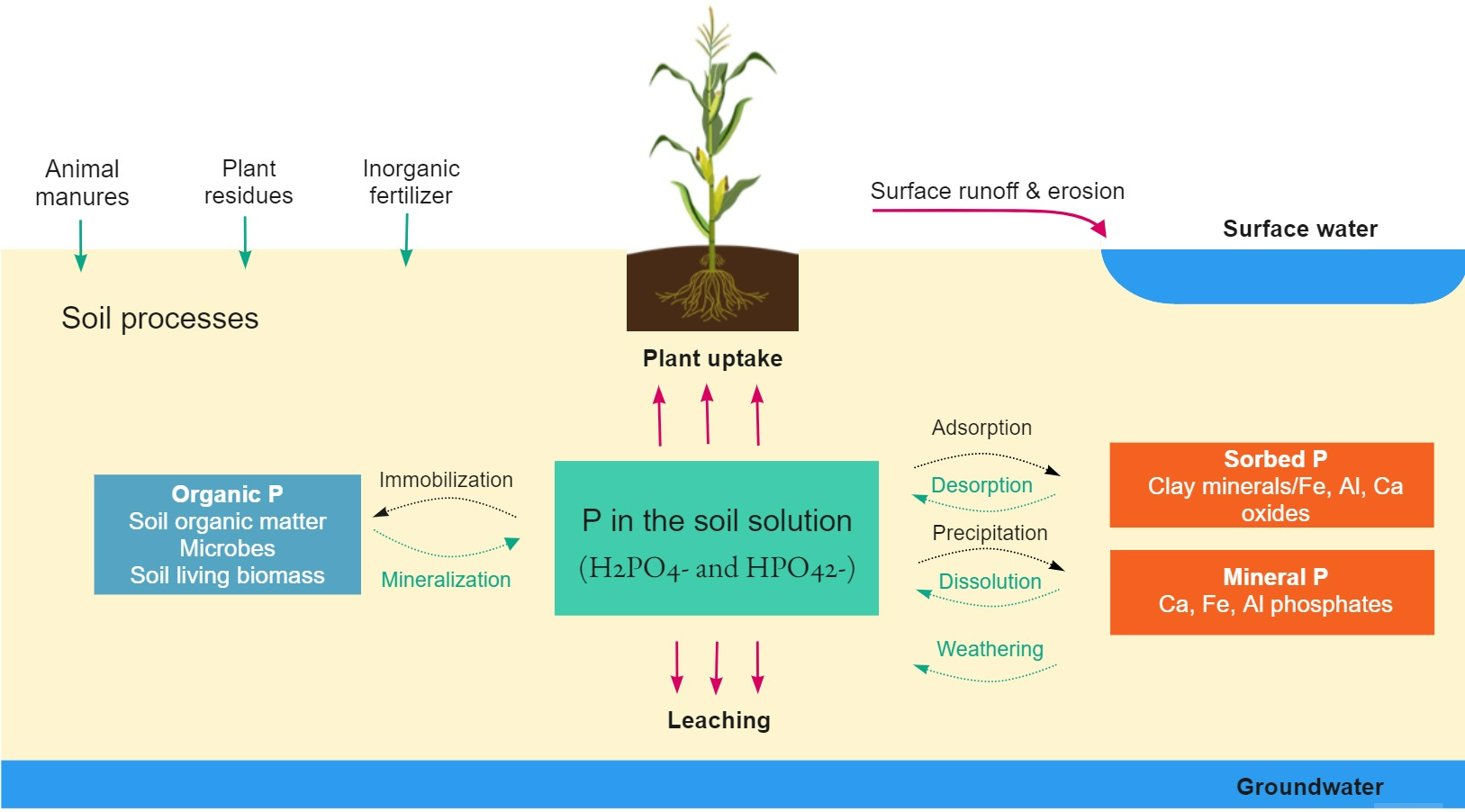
Figure 2:Phosphorous pathways and transformations in the soil. Adapted from Prasad & Chakraborty (2019).
The role of clay content and soil mineralogy¶
Inorganic P has high affinity with clay particles, iron (Fe), aluminum (Al) and calcium (Ca) oxides in the soil Prasad & Chakraborty, 2019Zak & Gelbrecht, 2007. Iron and aluminum phosphates predominate in acidic soils and sediments, while calcium phosphates are present in alkaline soils Ponnamperuma, 1972. In anaerobic conditions, due to the increase in pH in acidic soils and decrease in alkaline soils, insoluble iron, aluminum or calcium oxides are reduced to soluble forms (Fe2+, Al2+, Ca+), which can move readily in the soil solution Ponnamperuma, 1972. Adsorbed phosphorous is then released and becomes part of the soil solution. Clayey soils or soils with high concentrations of Fe, Al or Ca oxides, have a greater P adsorption capacity and therefore P availability will be larger when a bigger portion of the soil becomes saturated Prasad & Chakraborty, 2019. Riet et al. (2013) found that P values in rewetted peat- and clay-covered peatlands were up to 11.7 mg P-PO4/l, being higher in peat due to larger availability of iron-bond phosphorous. Under saturated conditions, manganese dioxide (Mn4+) is also reduced to soluble manganese ions (Mn2+). High concentrations of Fe2+and Mn2+ ions can be toxic for plants Ponnamperuma, 1972.
Mineralization and transport processes¶
Mineralization is the process through which nutrients present in organic matter (C, P, N, K) are converted into inorganic compounds easily available for plants. This process is intensified at higher soil moisture content, because soil microorganisms prefer wetter environments Whalen et al., 2001, but it slows down under anaerobic conditions because anaerobic bacteria work at a slower rate. Clearly, with higher organic matter content (i.e. peatlands), more available forms of P can be released into the soil Prasad & Chakraborty, 2019. Further, organic molecules like humic acids can hinder sorption and precipitation processes of phosphate, leading to more availability of soluble P in the soil Amery & Vandecasteele, 2015Prasad & Chakraborty, 2019. Zak & Gelbrecht (2007) found that the highest soluble P concentration in pore water (143 µM) was measured in highly decomposed peat, while negligible increases were found in slightly decomposed peat.
Surface runoff is the main hydrological pathway of phosphorus loss from soils to surface waters Prasad & Chakraborty, 2019, particularly in agricultural and livestock areas. Runoff water transports particulate P within eroded soil particles, and dissolved P. Diffusion of the soluble P depends greatly on the soil moisture content since soluble P moves through the soil pores by water, thus it can be more easily transported and lost by surface runoff when increasing groundwater levels Amery & Vandecasteele, 2015. Surface runoff during rainfall events is higher in saturated soils, and the risk of P losses increases in areas with a high P surplus like is the case of the Flemish region.
Nitrogen transformation¶
Just like phosphorus, nitrogen is an essential nutrient for crop growth, development and reproduction Moore et al., 1998. It is present in soils and sediments mainly as complex organic substances (which are not readily available for plant uptake), ammonium (NH4+), molecular nitrogen (N2), nitrite (NO2-), and nitrate (NO3-) Ponnamperuma, 1972. The transformations depend largely on microbial activity controlled by physical and chemical characteristics of the soil such us organic matter, temperature, soil moisture and pH. Ammonium and nitrate are soluble forms of nitrogen, readily available for plants. In anaerobic soils, the main transformations are the accumulation of NH4+, denitrification and nitrogen fixation Ponnamperuma, 1972. Most of the nitrogen is lost from the soil in the form of nitrogen gas; these nitrogen losses cause less soluble nitrogen to be available for plants. Figure 3 shows the main transformations occurring in nitrogen.
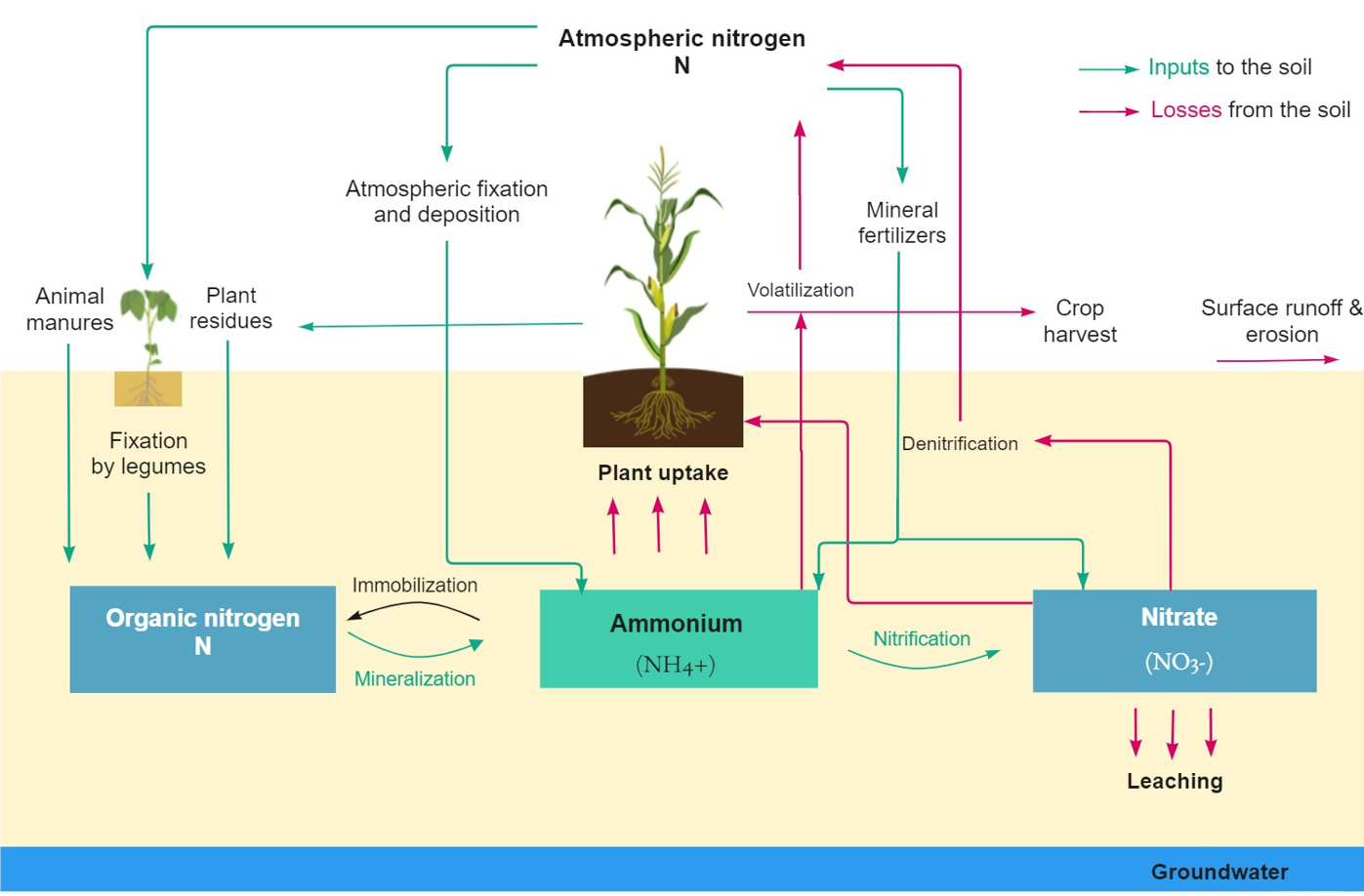
Figure 3:Nitrogen pathways and transformations. Adapted from ESN (2020).
Accumulation of ammonium¶
After increasing groundwater levels until soil saturation, microorganisms quickly deplete the remaining oxygen. Due to lack of oxygen, chemical transformation of organic nitrogen (mineralization) cannot go further in the conversion to NO3-, and consequently NH4+ accumulates in the soil Buresh et al., 2008. The fact that anaerobic bacteria have low nitrogen requirements also contributes to a faster ammonium release and accumulation Tusneem, 1971. Riet et al. (2013) reported that high ammonium release (4.8 mg N- NH4+/l) was observed in rewetted peatlands. Also, Zak & Gelbrecht (2007) found that the upper highly decomposed peat layer was mostly responsible for the high mobilization of ammonium after rewetting, due to availability of decomposable organic matter. Ammonia volatilization is another pathway of nitrogen loss from fertilized flooded soils with urea. High pH (7.5 to 10) and temperature favors the loss of added fertilizer through ammonia volatilization Buresh et al., 2008.
Denitrification and nitrogen fixation¶
Denitrifying bacteria use NO3- instead of oxygen as the oxidizing agent to transform nitrogen into nitrogen gas (N2or NO2), which finally escapes to the atmosphere Ponnamperuma, 1972. In presence of high nitrate availability (i.e. through fertilization, plant residues), incomplete denitrification due to shortage of oxygen can induce nitrous oxide (N2O) emissions Vroom et al., 2018IPV, 2022. Nitrous oxide has been found to be negligible in rewetted wetlands Vroom et al., 2018. In flooded soils, ammonium can be converted to nitrate in the thin upper aerobic layer and diffuse down to the anaerobic zone, where it is denitrified Tusneem, 1971.
The depletion of oxygen and high amount of dissolved organic carbon also promote the biological nitrogen fixation by cyanobacteria. This process occurs typically in paddy rice Buresh et al., 2008.
Other transformations¶
Carbon transformation¶
The main transformation of carbon in waterlogged conditions is the decomposition of the organic matter (e.g. carbohydrates) by soil microorganisms during respiration Ponnamperuma, 1972. This transformation is much slower than in aerobic conditions because the energy released in these transformations is much lower. Methane (CH4) is the typical end product of the anaerobic decomposition of organic matter, accompanied usually by smaller amounts of carbon dioxide (CO2), organic acids and hydrogen Ponnamperuma, 1972. CH4 emissions are one of the main concerns in rewetted peatlands because it is a highly polluting gas. In soils rich in organic matter, CO2 can accumulate in the porewater and potentially dissolve carbonates Zak & Gelbrecht, 2007.
Besides P, iron oxides are also bonded to organic carbon substances. The reduction of these oxides under anaerobic conditions releases dissolved organic carbon (DOC) in the soil solution Zak & Gelbrecht, 2007Harpenslager et al., 2015Maranguit et al., 2017.
Sulphate transformation¶
In very poorly drained soils, sulphate (SO42-) is reduced to sulphide (S2-) and sometimes hydrogen sulphide (H2S) Moore et al., 1998. H2S can react with heavy metals to produce insoluble sulphides, or provide hydrogen to photosynthetic sulfur bacteria Ponnamperuma, 1972. Zak & Gelbrecht (2007) identified that sulphate concentration increased sharply after rewetting, but had a rapid decrease in the upper highly decomposed peat horizon.
Effects on water quality and biodiversity¶
An increase of nutrient availability in the soil solution, especially phosphorous (P), can lead to contamination of local semi-aquatic ecosystems. This increase is insignificant agronomically because phosphorus and nitrogen are limiting nutrients for crop growth, but small concentrations in the water (20 μg/l) can already deteriorate aquatic ecosystems and reduce water quality Bomans et al., 2005. The amount of P release in the water is dependent on its availability in different forms in the soils, either in the organic matter, attached to iron (Fe), aluminum (Al) and calcium (Ca) particles, or as phosphate minerals. Also, the Fe/P ratio determines the export of P from eutrophic wetlands to nearby water bodies Zak et al., 2004. Soil nitrogen pollution due to nitrogen gases deposition is less probable under waterlogged conditions because emissions of contaminant gases such us ammonia and nitrous oxides are very small.
In the case of rewetting of peatlands, the removal of the highly decomposed top layer, has been considered as a mechanism to reduce carbon losses, P and N mobilization, and ultimately eutrophication problems Zak & Gelbrecht, 2007Harpenslager et al., 2015. Top soil removal can also avoid that fast-growing plants in nutrient rich water (e.g. cattail, reed) take over and decrease the biodiversity of the wetland Harpenslager et al., 2015. However, this method can be expensive Klimkowska et al., 2010. An alternative solution is the use of wetland plants to absorb excess nutrients and prevent nutrient diffusion and accumulation Vroom et al., 2018Geurts et al., 2020. This is explained in more detail in the next chapter (Potential of paludiculture crops in Flanders) .
- Harpenslager, S. F., van den Elzen, E., Kox, M. A. R., Smolders, A. J. P., Ettwig, K. F., & Lamers, L. P. M. (2015). Rewetting former agricultural peatlands: Topsoil removal as a prerequisite to avoid strong nutrient and greenhouse gas emissions. Ecological Engineering, 84, 159–168. 10.1016/j.ecoleng.2015.08.002
- Zak, D., & Gelbrecht, J. (2007). The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry, 85(2), 141–151. 10.1007/s10533-007-9122-2
- Johnston, A. E., Dawson, C. J., & Agricultural Industries Confederation. (2005). Phosphorus in agriculture and in relation to water quality. Agricultural Industries Confederation.
- Vroom, R. J. E., Xie, F., Geurts, J. J. M., Chojnowska, A., Smolders, A. J. P., Lamers, L. P. M., & Fritz, C. (2018). Typha latifolia paludiculture effectively improves water quality and reduces greenhouse gas emissions in rewetted peatlands. Ecological Engineering, 124, 88–98. 10.1016/j.ecoleng.2018.09.008
- Belle, J. (2021). Natte teelt voor waterkwaliteit Verkenning van de bijdrage van paludicultuur aan waterkwaliteitsverbetering in een Friese polder [Techreport]. Van Hall Larenstein University of applied sciences. https://betterwetter.nl/wp-content/uploads/2022/01/Natte-teelt-voor-waterkwaliteit-v1.1def.pdf